
Gone in a flash – What is Flash-Rusting and how can it be prevented?
Flash-rust inhibitors from ASCOTEC can be used to prevent corrosion and flash-rusting in waterborne coatings. This article explains how they work and the benefits of them.

With the outbreak of the COVID-19 pandemic, the world and its factories are slowing down. This unexpected event is bringing many newfound challenges and exacerbating others. One of those challenges is corrosion – a silent enemy that by some estimates costs the global economy several billions of pounds per year.
Corrosion, the electrochemical reaction of metals with their environment over time, has a detrimental effect on the metal components it affects and it must be avoided at all costs. For the production of metal parts, this means that the metal surface must be protected during the entire production process which includes all metalworking operations as well as during storage and transport.
In these uncertain times, manufacturers must protect themselves from potential delays at each stage of production especially whilst the relative timescales are changing and unpredictable. Furthermore, many metal tanks, containers, and pipes which are usually flowing can be left stagnant – again opening these static systems to the negative effects of corrosion.
It is often unfeasible to apply a permanent protective coating such as a paint or a plastic coating to metal parts to prevent corrosion. This would be cost-prohibitive and there may be difficulties in removing the coating once the parts or systems are again required. The preferred solution is temporary in nature: it should be easily applied and just as easily removed from the metal surface after treatment. Or ideally, it should not need to be removed at all. For example, oil and grease-based corrosion preventatives are examples of the former. In this article, we will explore the benefits of novel aqueous corrosion inhibitors for the protection of metals, which have the advantage that they do not leave a greasy layer and do not need to be removed at all.
Developed by ASCOTEC®, ASCOTRAN®-L is designed as a complete replacement for oil-based corrosion preventatives for the protection of metal equipment and components. In contrast to oil-based systems, it does not leave a greasy layer. ASCOTRAN®-L works by forming a monomolecular protective layer on the metal surface which highly hydrophobic (Figure 1). The molecular layer inhibits the electrochemical reactions on the surface of the metal that cause corrosion. A welcome side effect of the monomolecular layer is that it is dry to the touch, unlike its greasy counterparts.

Figure 1. ASCOTRAN® works by forming a hydrophobic molecular layer on the metal surface, providing metal protection both during metal working operations and storage or transport.
For the temporary protection of metal parts, ASCOTRAN®-L can be applied by spraying, dipping, or by addition into the last metalworking bath. For optimal efficiency, the pH of the solution should be neutral or slightly alkaline and after drying, a hydrophobic protective layer is formed.
The efficacy of ASCOTRAN®-L on protection duration was evaluated according to ASTM D2247. In this test, specimens of copper (99%) and mild-steel were immersed for several minutes in tap water containing the corrosion inhibitor and placed in a climatic chamber at 25°C and 100% relative humidity. The effect of the extreme humidity is an acceleration of the onset of corrosion that could be expected in real-world conditions. The maximum exposure time is determined by observing the onset of corrosion through a colour change. The results are shown in Table 1.

Table 1. Humidity test results of ASCOTRAN®-L according to ASTM D2247. ASCOTRAN®-L significantly delays the onset of corrosion on copper and mild-steel allowing safe storage between metalworking operations.
In the case of the control samples where no ASCOTRAN®-L was present in the solution, both copper and mild-steel were found to corrode within a day under these harsh conditions. By the addition of small amounts of corrosion inhibitor, the resistance to corrosion was improved significantly. For copper, a protection duration of 1000 hours (6 weeks) could be achieved by adding 2% of ASCOTRAN®-L.
In the case of mild-steel, a larger amount of corrosion inhibitor was required to achieve lasting protection (up to 10%). Importantly, the metals can still be painted over in their protected state using conventional paint systems. This is a unique feature of the aqueous corrosion inhibitor and is due to the monomolecular nature of the protective layer.
For applications where the metal is in long term contact with a static solution (e.g. in pipes, tanks, containers), ASCOTRAN®-L can be added directly into the liquid.
Table 2 shows the benefit of a small addition of ASCOTRAN®-L to aqueous solutions by means of a standardised immersion test. The investigated metals included mild-steel, aluminium (99%), copper (99%), and brass.
In this experiment, several metallic specimens were immersed in tap-water containing a low percentage of ASCOTRAN®-L corrosion inhibitor. The solutions were kept at room temperature and the time was noted at which the onset of corrosion was visible.
Remarkably, using very low addition levels of ASCOTRAN®-L (< 0.3 %), the onset of corrosion was delayed from days to years for all the metals investigated. Translating this result to the real world, one could safely restart these systems after many months of standstill when making use of a small amount of ASCOTRAN®-L, potentially preventing thousands of pounds of damage due to corrosion.
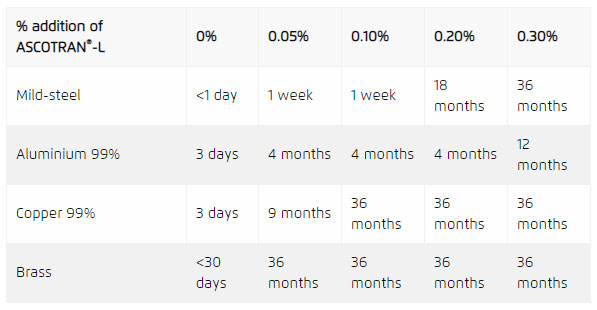
Table 2: Immersion testing of ASCOTRAN®-L.The timescale of the onset of corrosion is extended from days to months/years by the addition of ASCOTRAN®-L
Finally, ASCOTRAN®-L is free of inorganic materials such as borates, phosphates, molybdates, silicates, and nitrites which has both environmental and labelling benefits. For example, ASCOTRAN®-L is approved for indirect food contact (substances for food contact cleaners according to decree 08/09/99).
Small additions of ASCOTRAN®-L can help to dramatically improve corrosion resistance.
Please call our technical sales team on 01827 314151 to see how we can help you keep your metal parts and systems safe from corrosion.




Flash-rust inhibitors from ASCOTEC can be used to prevent corrosion and flash-rusting in waterborne coatings. This article explains how they work and the benefits of them.

In the ever-evolving world of cosmetics, the quest for vibrant, eye-catching colours that are both ethically sourced and environmentally friendly has led to significant innovations in pigment technology. GEOTECH’s latest offer, the Geopearl® C Crystal Super Deep Pink and Purple pigments, represents a leap forward in combining high-impact colour with a commitment to cruelty-free and vegan product formulations.

How the spherical nature of glass bubbles can be used to produce high Total Solar Radiance to achieve cooler, more energy efficient buildings.