
Fire Retardant Additives for Thermal Runaway
Expandable graphite and Quarzwerke minerals can be used to enhance flame-retardant systems for EV safety.

Foam is a dispersion of gas in a continuous solid or liquid medium. It is an example of a colloidal system. Many examples of foam can be seen in everyday life as a result of natural or industrial processes e.g. whipped cream or soap suds. Foam, however, is not always desirable and causes issues in a variety of industrial applications including food processing, textiles and detergents. Here, it can impact the quality of the finished product, interfere with the manufacturing process or create potentially hazardous working conditions. Clearly, then, it is necessary to implement a foam control procedure for these processes. Options include mechanical and thermal methods for the destruction of foam. The capital expenditure required for this can be prohibitive though. A more cost-effective approach can be the use of defoamer additives.
When choosing a defoaming additive, some properties must be considered. Defoamers meeting all the criteria found in Figure 1 can be made from dispersions of hydrophobic silica in oil. A variety of different oils and silicas can be used to achieve the desired results.

Figure 1: Some of the key desirable properties of defoamers from a formulation, chemical and end-user point of view.
It is difficult to define a general, quantitative mechanism for defoaming. This is because there are a very large number of different defoamers and foam systems. Qualitatively, however, it’s possible to divide the defoaming of aqueous systems into four main steps:
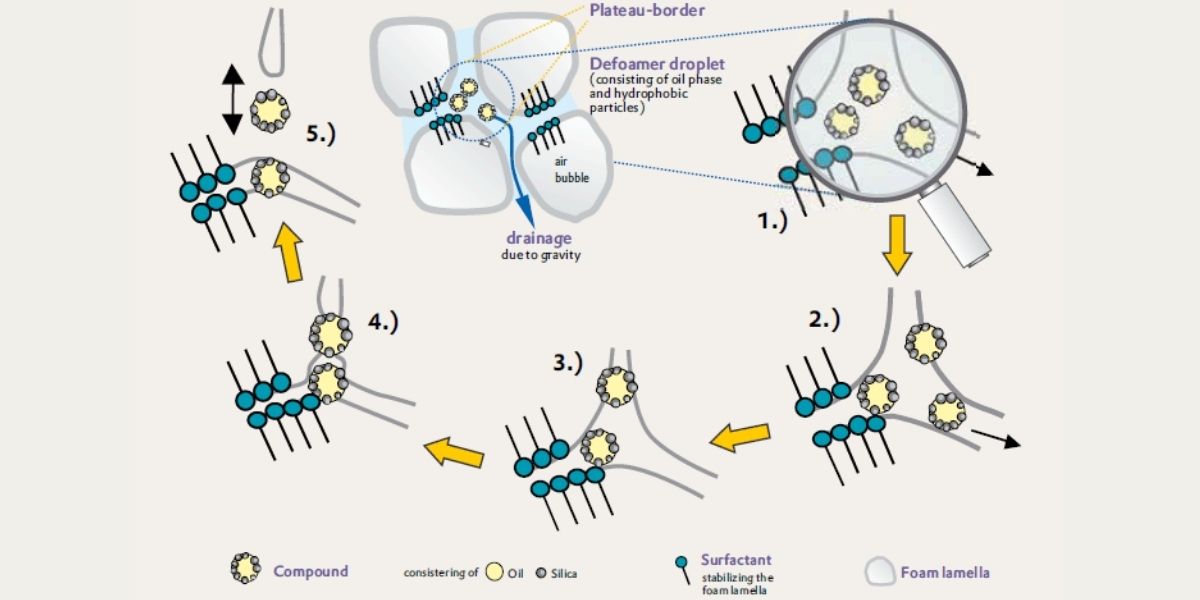
Figure 2: A graphical representation of the defoaming mechanism of silica/oil droplets in an aqueous form.
For more information on the use of defoamers in a formulation see our earlier article on defoamers in applications.
Defoamer performance is majorly influenced by the ability of the oil droplet to enter and spread around the foam system. The faster, and more evenly the oil spreads, the more likely the defoamer is to be effective. A balance must be struck, as smaller oil droplets will enter and spread more easily but are less likely to be effective in bridging and dewetting foam lamellae. The rate at which the foam film ruptures also impacts the actual effectiveness of the defoamer. This is because practical systems are not in a state of equilibrium and foam bubbles continuously form due to mechanical mixing.
Some of the major defoamer categories are listed below:
The introduction of hydrophobic particles produces a synergistic effect with defoamer oils. These dispersions of hydrophobic particles in oil are substantially more effective than either of the individual components, used in isolation. Oil droplets form a pseudoemulsion film within the foam system consisting of oil/water/air. The addition of hydrophobic particles causes the rapid destabilisation of this film as they drastically increase the depth and rate of penetration of the defoamer droplets into the foam lamellae (pin-effect).
The hydrophobic particles must be present on the surface of the oil droplet, rather than suspended within. The larger and rougher the hydrophobic particles are and the amount they protrude from the oil droplets, the more effective the defoamer will be in destabilising the pseudoemulsion film. Larger particles, however, mean fewer particles, and poor distribution. They are also more prone to sedimentation, during storage. Application tests are therefore necessary to determine the optimum oil/silica parameters for a particular system.
The use of hydrophobic silica as solids in defoamers has a number of advantages over other alternatives such as waxes or metal soaps. Primarily, they have a defined particle size and a rough, porous surface. This allows the formulator to make an easy and informed decision when selecting a grade, whilst ensuring finished product consistency. They also have the added advantage of being unhindered by temperature or water hardness sensitivity. Silicas can also act as anti-settling agents, to help stabilise defoamer formulations. Defoamer solutions can be dried out to powder form using carrier silicas. Evonik offer a number of hydrophobically treated silica grades – SIPERNAT (precipitated silica) & AEROSIL (fumed silica).
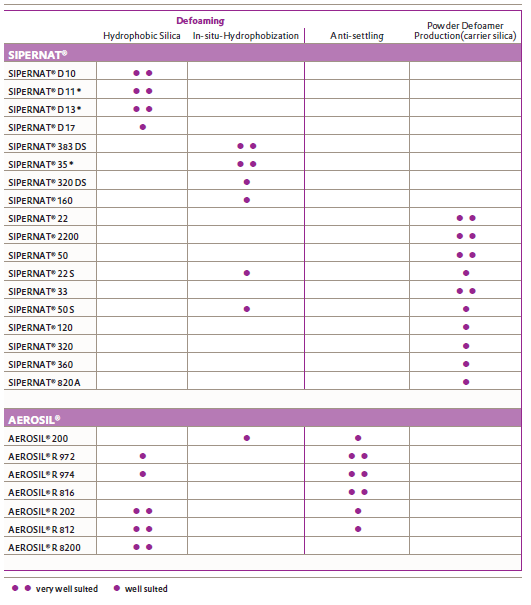
Table 1: Using the above grade selector, the best Aerosil and Sipernat grades can be selected for the appropriate purpose.
There are some important considerations when using hydrophobic silica in defoamer formulations. Due to attractive interactions and their large surface areas, silica particles tend to form agglomerates when stored. The more finely divided the silica, the greater the tendency to agglomerate. As a result, AEROSIL grades tend to agglomerate more than SIPERNAT grades. Dispersion within the selected oil is therefore very important. The more viscous the oil, the more important this dispersion step is. Standard low shear mixing will not be sufficient. Higher shear mixers, such as rotor-stator units will be required in order to achieve adequate dispersion.
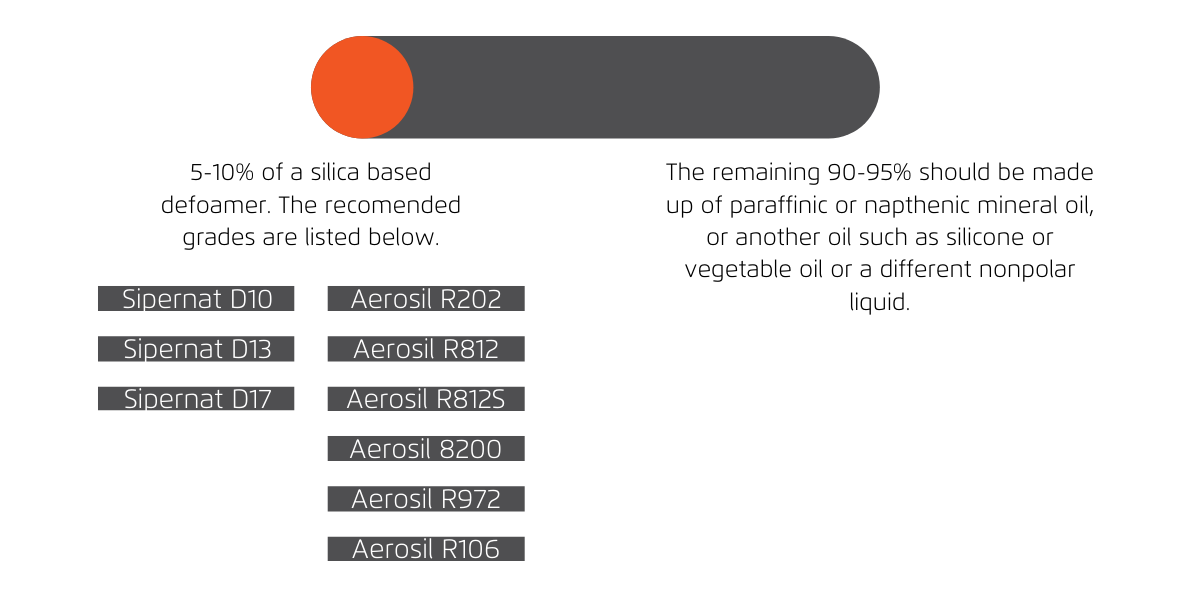
Figure 3: Silicas are only required at around 5-10% within a formulation and there are plenty of Evonik Silicas that can be used depending on the formulation. For a more in depth starting point formulation for your specific needs, get in contact with us and one of our technical team would be happy to assist you.
Defoaming is a complex process, with a wide variety of factors effecting the efficacy of defoamer products. There are a number of ways of handling foam in industrial applications, both mechanically and chemically. The combination of oils and hydrophobic particles, such as speciality silicas, is well-documented and effective for improving the performance of chemical defoamers without having to invest in expensive process machinery. For further insight and for recommendations for your formulation, contact your account manager or call us to discuss your requirements.



Expandable graphite and Quarzwerke minerals can be used to enhance flame-retardant systems for EV safety.

High Temperature Expandable Graphite from LUH is able to be used in thermoplastics without expanding during the extrusion process.

Micaceous Iron Oxide (MIO) is a naturally occurring iron oxide used in protective coatings due to a lamellar/platy morphology that allows it to form a barrier towards the ingress of water.